Exciting news! Our group published a new Angew. Chem. paper on August 1st: Jiahua Wang, Junyou Wang*, Peng Ding, Wenjuan Zhou, Yuehua Li, Markus Drechsler, Xuhong Guo*, Martien A. Cohen Stuart*. Beating Brine Supramolecular Crosslinker Gives Salt Resistent PIC Micelles and Improved MRI Contrast Agents. Angew. Chem. Int. Ed. 2018, DOI: 10.1002/anie.201805707.
Polyion complex micelles have attracted increasing attention in recent years because of their perceived potential as carriers for drugs, enzymes, and DNA. However, the fact that charge provides the driving force makes polyelectrolyte micelles very sensitive to ionic strength: salt screens the electrostatic interaction between charged blocks and strongly affects the coacervation and the stability of the coacervate complexes that constitute the micellar core. Typically, PIC micelles hardly tolerate physiological salt concentration, which seriously limits their application in biomedical contexts.
Previously, we have reported a novel type of three-component PIC micelles prepared from a polycationic-neutral diblock copolymer and an anionic supramolecular, reversible coordination polyelectrolyte (Angew. Chem. Int. Ed. 2007, 46, 1807 –1809). The latter was obtained by coordination between various metal ions and a bis-ligand (L2) containing two dipicolinic acid (DPA) groups connected by a tetra-ethylene oxide spacer (4EO). Unlike micelles formed by synthetic polyelectrolytes only, such coordination polyelectrolytes bring hundreds of metal ions in the micellar core, resulting in novel functional properties, e.g., magnetic and fluorescent properties. Regrettably, it was the poor stability against salt that hampered their practical application, particularly for first-row transition metals. The reason for this may be that the combination of bis-ligand (L2) with these ions can only form linear coordination polymers, producing highly mobile, liquid-like coacervates.
In the present study, we therefore introduce, as an alternative for L2, a triple ligand with three DPA groups grafted on a benzene ring (L3). When L3 is added, it creates cross-links between the linear metal-L2 coordination structures, and then produces reversible network coordination polymers that, as we have demonstrated, are less easily destroyed by salt. The cross-link density can be simply controlled by varying the L3/L2 ratio, so that we establish a method to prepare micelles with adjustable composition, tunable and enhanced salt stability, and hopefully other improved properties.
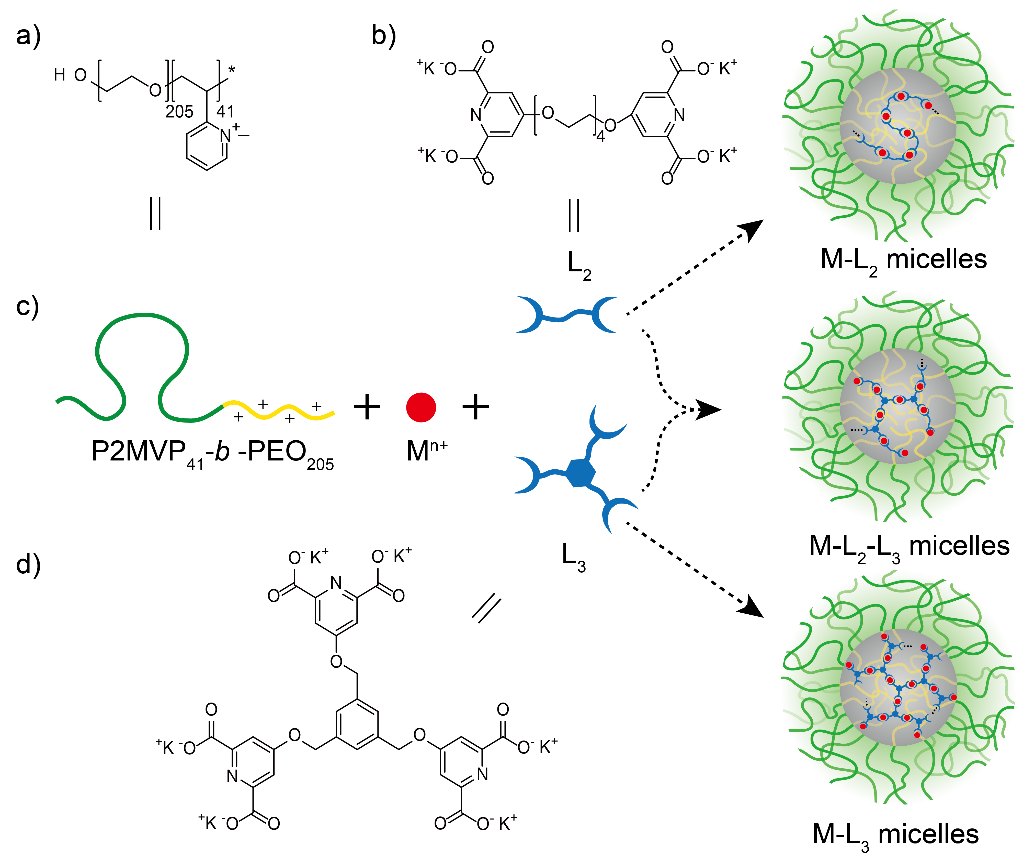
Figure 1. a) Structure of P2MVP41-b-PEO205. b) Structure of bis-ligand L2. c) Illustration of the formation of M-L2-L3 micelles. d) Structure of tris-ligand L3.
We also found that the longitudinal relaxivity of Mn-containing micelles, which is around 5.6 mM-1 s-1 for Mn-L2, increases with increasing L3 fraction, initially in a linear fashion but eventually levelling off around 60% of L3. Pure Mn-L3 micelles display the highest relaxivity of 10.8 mM-1 s-1, which is higher than most of the reported Mn-based contrast agent.
Link for paper: https://onlinelibrary.wiley.com/doi/10.1002/anie.201805707